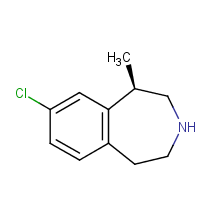
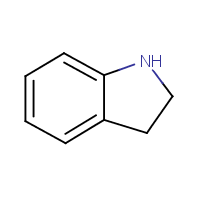
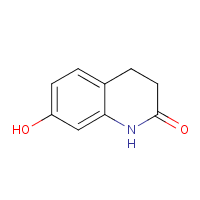
![]()
The Claimed Intermediate Online Database and Reports cover Process Patents for Marketed Pharmaceutical Drugs and the Intermediates used therein.

• Unique Online Database allows Intermediate/API/INN text and structure searching
• Includes API/INNs in at least one major Market
• Locates Drug Synthesis often buried in a Plethora of Patents
• Provides targeted Patent data in a Visual form
• Drug syntheses obtained by analysing Patent documents

• Subscription-based, no additional hit costs
• Save on patent searching/analysis
• Only new applications, no repeated equivalents
• Reduce manpower cost
• Informs Commercial Synthesis profitability
• 875+ Drugs • 3850+ Patents • 25,000+ Structures • 4000+ Records with 1150+ Claimed Intermediates • 1250+ Patentees including Joint Patentees &c
Top Database Records:
Try the Free Quarterly Report in the Shop.